Mo6+ activated multimetal oxygen-evolving catalysts†
Abstract
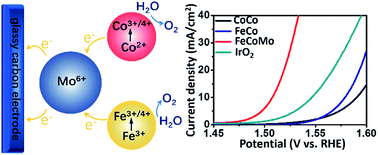
Water splitting is key to electrically-powered chemical fuel synthesis, but the slow kinetics of the oxygen evolution reaction (OER) hinder the wider promotion of such technology. Several first-row (3d) transition metal-based catalysts have been developed for the OER; however, these catalysts still require operating voltages that lie well above the fundamental thermodynamic potential. Here, we report high-valence metal molybdenum (Mo6+) modulated 3d metal (oxy)hydroxides. The obtained multimetal FeCoMo based OER catalysts require an overpotential of 277 mV to reach the current density of 10 mA cm−2 on the glassy carbon electrode, and there was no evidence of degradation for about 40 hours of stability testing. The catalysts stay in their amorphous phases, potentially with atomically homogenous metal distribution. The in situ X-ray adsorption analysis unambiguously reveals the tuned electronic structures of the 3d metals owing to Mo6+, further demonstrating the modification effect of a high-valence metal for designing highly-efficient OER catalysts.


 Please wait while we load your content...
Please wait while we load your content...