Piezochromism and hydrochromism through electron transfer: new stories for viologen materials†
Abstract
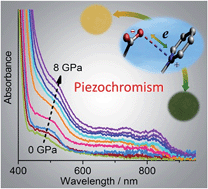
While viologen derivatives have long been known for electrochromism and photochromism, here we demonstrated that a viologen-carboxylate zwitterionic molecule in the crystalline state exhibits piezochromic and hydrochromic behaviors. The yellow crystal undergoes a reversible color change to red under high pressure, to green after decompression, and finally back to yellow upon standing at ambient pressure. Ultraviolet-visible spectroscopy, X-ray photoelectron spectroscopy, electron paramagnetic resonance X-ray diffraction and DFT calculations suggested that the piezochromism is due to the formation of radicals via pressure-induced electron transfer from carboxylate to pyridinium, without a crystallographic phase transition. It was proposed that electron transfer is induced by pressure-forced reduction of intermolecular donor–acceptor contacts. The electron transfer can also be induced by dehydration, which gives a stable green anhydrous radical phase. The color change is reversible upon reabsorption of water, which triggers reverse electron transfer. The compound not only demonstrates new chromic phenomena for viologen compounds, but also represents the first example of organic mechanochromism and hydrochromism associated with radical formation via electron transfer.


 Please wait while we load your content...
Please wait while we load your content...