Spin-crossover and high-spin iron(ii) complexes as chemical shift 19F magnetic resonance thermometers†
Abstract
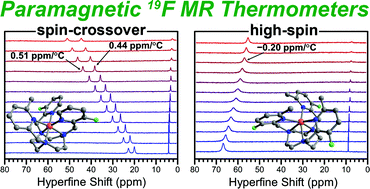
The potential utility of paramagnetic transition metal complexes as chemical shift 19F magnetic resonance (MR) thermometers is demonstrated. Further, spin-crossover FeII complexes are shown to provide much higher temperature sensitivity than do the high-spin analogues, owing to the variation of spin state with temperature in the former complexes. This approach is illustrated through a series of FeII complexes supported by symmetrically and asymmetrically substituted 1,4,7-triazacyclononane ligand scaffolds bearing 3-fluoro-2-picolyl derivatives as pendent groups (Lx). Variable-temperature magnetic susceptibility measurements, in conjunction with UV-vis and NMR data, show thermally-induced spin-crossover for [Fe(L1)]2+ in H2O, with T1/2 = 52(1) °C. Conversely, [Fe(L2)]2+ remains high-spin in the temperature range 4–61 °C. Variable-temperature 19F NMR spectra reveal the chemical shifts of the complexes to exhibit a linear temperature dependence, with the two peaks of the spin-crossover complex providing temperature sensitivities of +0.52(1) and +0.45(1) ppm per °C in H2O. These values represent more than two-fold higher sensitivity than that afforded by the high-spin analogue, and ca. 40-fold higher sensitivity than diamagnetic perfluorocarbon-based thermometers. Finally, these complexes exhibit excellent stability in a physiological environment, as evidenced by 19F NMR spectra collected in fetal bovine serum.

 Please wait while we load your content...
Please wait while we load your content...