A family of cis-macrocyclic diphosphines: modular, stereoselective synthesis and application in catalytic CO2/ethylene coupling†
Abstract
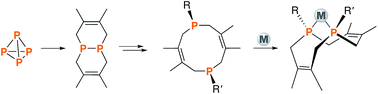
A family of cis-macrocyclic diphosphines was prepared in just three steps from white phosphorus and commercial materials using a modular synthetic approach. Alkylation of bicyclic diphosphane 3,4,8,9-tetramethyl-1,6-diphosphabicyclo(4.4.0)deca-3,8-diene, or P2(dmb)2, produced phosphino-phosphonium salts [R-P2(dmb)2]X, where R is methyl, benzyl and isobutyl, in yields of 90–96%. Treatment of these salts with organolithium or Grignard reagents yielded symmetric and unsymmetric macrocyclic diphosphines of the form cis-1-R-6-R′-3,4,8,9-tetramethyl-2,5,7,10-tetrahydro-1,6-DiPhospheCine, or R,R′-DPC, in which R′ is methyl, cyclohexyl, phenyl or mesityl, in yields of 46–94%. Alternatively, symmetric diphosphine Cy2-DPC was synthesized in 74% yield from the dichlorodiphosphine Cl2P2(dmb)2. As a first application, these cis-macrocyclic diphosphines were used as ligands in the nickel-catalyzed synthesis of acrylate from CO2 and ethylene, for which they showed promising catalytic activity.
- This article is part of the themed collection: ISACS17: Challenges in Chemical Renewable Energy


 Please wait while we load your content...
Please wait while we load your content...