Watasemycin biosynthesis in Streptomyces venezuelae: thiazoline C-methylation by a type B radical-SAM methylase homologue†
Abstract
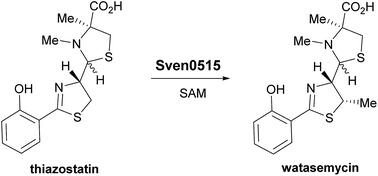
2-Hydroxyphenylthiazolines are a family of iron-chelating nonribosomal peptide natural products that function as virulence-conferring siderophores in various Gram-negative bacteria. They have also been reported as metabolites of Gram-positive Streptomyces species. Transcriptional analyses of Streptomyces venezuelae ATCC 10712 revealed that its genome contains a putative 2-hydroxyphenylthiazoline biosynthetic gene cluster. Heterologous expression of the gene cluster in Streptomyces coelicolor M1152 showed that the mono- and dimethylated derivatives, thiazostatin and watasemycin, respectively, of the 2-hydroxyphenylthiazoline enantiopyochelin are two of its metabolic products. In addition, isopyochelin, a novel isomer of pyochelin containing a C-methylated thiazolidine, was identified as a third metabolic product of the cluster. Metabolites with molecular formulae corresponding to aerugine and pulicatins A/B were also detected. The structure and stereochemistry of isopyochelin were confirmed by comparison with synthetic standards. The role of two genes in the cluster encoding homologues of PchK, which is proposed to catalyse thiazoline reduction in the biosynthesis of enantiopyochelin in Pseudomonas protegens, was investigated. One was required for the production of all the metabolic products of the cluster, whereas the other appears not to be involved in the biosynthesis of any of them. Deletion of a gene in the cluster encoding a type B radical-SAM methylase homologue abolished the production of watasemycin, but not thiazostatin or isopyochelin. Feeding of thiazostatin to the mutant lacking the functional PchK homologue resulted in complete conversion to watasemycin, demonstrating that thiazoline C-methylation by the type B radical-SAM methylase homologue is the final step in watasemycin biosynthesis.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners


 Please wait while we load your content...
Please wait while we load your content...