Microfluidic synthesis of α-ketoesters via oxidative coupling of acetophenones with alcohols under metal-free conditions†
Abstract
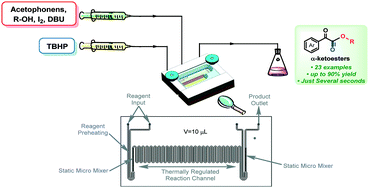
An efficient and novel method for the synthesis of α-ketoesters has been developed via oxidative coupling of acetophenones with alcohols under TBHP/I2/DBU conditions in a microfluidic chip reactor, which has a wide substrate scope, uses a lower dosage of iodine and affords higher product yields in only a few seconds. Moreover, a scale-up continuous flow system is also feasible and the plausible reaction mechanism has been proposed based on a series of control experiments.


 Please wait while we load your content...
Please wait while we load your content...