A direct strategy to synthesize hybrid benzothiazole–carbamate moieties via O-acylation of phenols under metal–organic framework catalysis†
Abstract
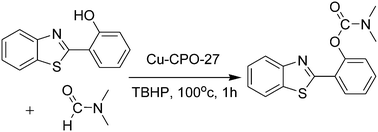
Carbamates containing a benzothiazole moiety were synthesized via cross-dehydrogenative coupling of phenols with N,N-disubstituted formamides utilizing the metal–organic framework Cu-CPO-27 as a productive and recyclable heterogeneous catalyst. These hybrid structures would combine the benefits from both carbamates and benzothiazoles in terms of biological and pharmaceutical activities. The Cu-CPO-27 framework exhibited better catalytic efficiency for the synthesis of carbamates than various MOFs. It was feasible to recover and reuse the catalyst without an appreciable deterioration in catalytic efficiency. To the best of our knowledge, this is the first heterogeneous catalytic protocol towards the generation of a hybrid carbamate–benzothiazole skeleton via direct coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...