Expedient Diels–Alder cycloadditions with ortho-quinodimethanes in a high temperature/pressure flow reactor†
Abstract
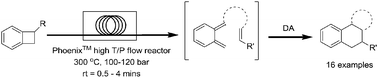
We describe herein Diels–Alder cycloadditions enabled by the efficient ring-opening of benzocyclobutenes and benzothiophene-2,2-dioxides using a high temperature/pressure flow reactor. The resultant ortho-quinodimethanes were generated and subsequently reacted with a wide range of dienophiles to provide complex heterocyclic ring systems. The use of flow technology allowed these reactions to be completed within minutes using conventional, readily removed solvents.


 Please wait while we load your content...
Please wait while we load your content...