Singlet oxygen oxidations in homogeneous continuous flow using a gas–liquid membrane reactor†
Abstract
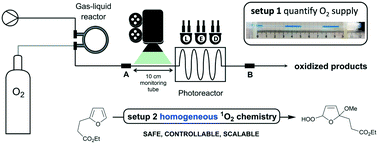
A flow chemistry reactor system for scalable photooxygenation reactions was developed using a gas–liquid reactor as key feature. Herein oxygen gas is dissolved under pressure in the reaction mixture to give a homogeneous flow regime prior to irradiation. The system enables safe mass transport of oxygen in an accurate manner simply by adjusting liquid flow rate, membrane temperature, and gas pressure as concluded after characterization of reactor behaviour. Quantification of oxygen supply as function of liquid flow rate and membrane temperature showed that 50 mM and 58 mM of oxygen were supplied to methanol and acetonitrile at 1 mL min−1 flow rate and 110 °C membrane temperature. With 100 mM measured at 90 °C, dichloromethane was most effective for oxygen uptake. Photooxidation of a model system, the furan derivative ethyl 3-(2-furyl)propanoate, was elaborated to validate the system for singlet oxygen chemistry, with reactor parameters being further optimized for maximum conversion. Scope of the system was demonstrated by performing a set of representative photochemical oxidations, including reaction with citronellol as first step in the synthesis of rose oxide.


 Please wait while we load your content...
Please wait while we load your content...