Synthesis of polymeric nano-objects of various morphologies based on block copolymer self-assembly using microporous membranes†
Abstract
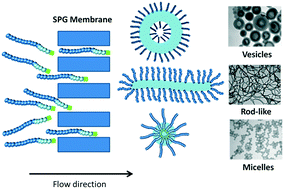
Membrane emulsification entails the use of two immiscible liquids for preparation of oil-in-water or water-in-oil emulsions of well-defined droplet size. In this work, we have developed a novel approach for preparation of polymeric nano-objects of various morphologies based on self-assembly of diblock copolymers in tandem with microporous membrane technology (Shirasu porous glass (SPG) membranes). Diblock copolymers poly(oligoethylene glycol acrylate)-b-poly(styrene) (POEGA-b-PSt) comprising different block lengths were prepared by RAFT polymerization. Self-assembly using SPG membrane technology was subsequently conducted by first dissolving the polymer in tetrahydrofuran, followed by continuous passage of this copolymer solution through the membrane pores into the aqueous phase. The results indicate that it is possible to conveniently tune the nano-object morphology via the process parameters of pressure and pore size for a given formulation. The technique also holds promise with regards to high throughput, in particular in the case of vesicles.


 Please wait while we load your content...
Please wait while we load your content...