Solvent-free coupling of aryl halides with pyrroles applying visible-light photocatalysis
Abstract
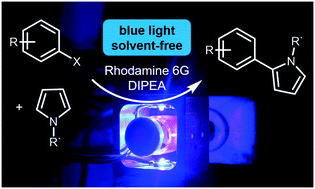
A novel reactor for solvent-free, visible-light-driven photocatalytic transformations was developed. By rotation of the reaction vessel, the reaction mixture forms a thin film, which allows efficient excitation of the photocatalyst. The reactor was used for the coupling of aryl halides with pyrrole derivatives and phosphites, applying rhodamine 6G as the photocatalyst and DIPEA as the sacrificial electron donor. The necessary amounts of photocatalyst, trapping reagent, and sacrificial electron donor were reduced significantly compared to those for literature known reactions in solution, while isolating the products in moderate to good yields. In general terms, this solvent-free methodology is an interesting alternative to solution photocatalysis due to the presence of high mole fractions of trapping reagent, the exclusion of by-products formed with the solvent, and the reduction of toxic solvent waste.


 Please wait while we load your content...
Please wait while we load your content...