Temperature-induced deactivation mechanism of ZnFe2O4 for oxidative dehydrogenation of 1-butene
Abstract
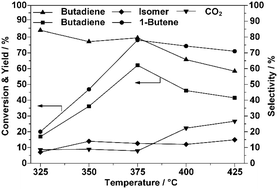
This paper describes the observation of an irregular decrease in 1-butene conversion during oxidative dehydrogenation over ZnFe2O4 in the production of 1,3-butadiene upon increasing the reaction temperature above 400 °C. Mono and multi-pulse adsorption and reoxidation were developed to determine the reaction mechanism that led to this phenomenon. Adsorbed species were found to be generated by 1-butene adsorption, which blocked the active sites, and with higher reaction temperatures, the surface reconstruction of the catalyst could lead to catalyst deactivation. Temperature-programmed oxidation revealed the correlation between different adsorbed species and the corresponding altered active sites. A temperature-induced surface reconstruction mechanism to explain the possible deactivation was then proposed and confirmed with X-ray photoelectron spectroscopy. The original spinel surface structure of the catalyst was transformed into cubic cells formed by Zn2+ and Fe2+ after the initiation of adsorption at high temperatures, which resulted in its final deactivation.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...