Continuous flow synthesis of aryl aldehydes by Pd-catalyzed formylation of phenol-derived aryl fluorosulfonates using syngas†
Abstract
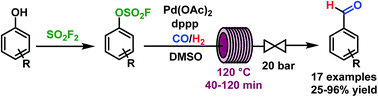
This communication describes the palladium-catalyzed reductive carbonylation of aryl fluorosulfonates (ArOSO2F) using syngas as an inexpensive and sustainable source of carbon monoxide and hydrogen. The conversion of phenols to aryl fluorosulfonates can be conveniently achieved by employing the inexpensive commodity chemical sulfuryl fluoride (SO2F2) and base. The developed continuous flow formylation protocol requires relatively low loadings for palladium acetate (1.25 mol%) and ligand (2.5 mol%). Good to excellent yields of aryl aldehydes were obtained within 45 min for substrates containing electron withdrawing substituents, and 2 h for substrates containing electron donating substituents. The optimal reaction conditions were identified as 120 °C temperature and 20 bar pressure in dimethyl sulfoxide (DMSO) as solvent. DMSO was crucial in suppressing Pd black formation and enhancing reaction rate and selectivity.


 Please wait while we load your content...
Please wait while we load your content...