Synthesis of substituted pyrazines from N-allyl malonamides†
Abstract
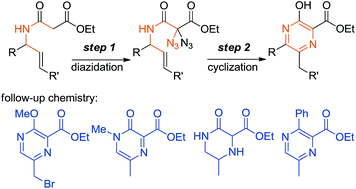
The synthesis of pyrazines is described using a sequence that begins with the diazidation of N-allyl malonamides followed by thermal or copper-mediated cyclization. The pyrazine products possess an ester and a hydroxy group at 2- and 3-positions of the heterocyclic core, while alkyl- and aryl groups may be introduced at the other positions. We also show how to modify the pyrazines obtained with our method; examples regarding alkylations, side-chain brominations, hydrogenations and cross-couplings are presented.


 Please wait while we load your content...
Please wait while we load your content...