An arginine functionalized magnetic nano-sorbent for simultaneous removal of three metal ions from water samples†
Abstract
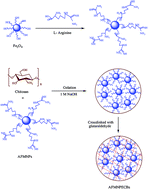
The feasibility of using eco-friendly biodegradable arginine functionalized magnetic nanoparticle entrapped chitosan beads (AFMNPECBs) for simultaneous removal of three metal ions from water samples was evaluated under different conditions: concentration, pH, temperature and time. A novel approach was developed to synthesize AFMNPECBs for the removal of Cu(II), Co(II) and Ni(II) ions from aqueous solution. The synthesized AFMNPECBs were characterized by various techniques including Fourier transform infrared (FTIR), thermogravimetric analysis (TGA), vibrating sample magnetometer (VSM) and Brunauer–Emmett–Teller (BET). The adsorption kinetics study for Cu(II), Co(II) and Ni(II) ions on AFMNPECBs at pH 6 confirmed that it followed a pseudo second order kinetic model for all the metal ions. The adsorption isotherm data of the metal ions was fitted well with the Freundlich isotherm model. The maximum removal by AFMNPECBs was found to be 86, 82, and 71% and followed an order Cu(II) > Co(II) > Ni(II), respectively, with the maximum adsorption capacity being 172.4, 161.2, and 103.0 mg g−1, correspondingly. The AFMNPECBs exhibited highly effective adsorption for the removal of Cu(II), Co(II) and Ni(II) ions as they could be regenerated four times inexpensively by 0.1 M EDTA solution retaining 70% of the adsorption capacity.


 Please wait while we load your content...
Please wait while we load your content...