Monte Carlo study of chemical reaction equilibria in pores of activated carbons†
Abstract
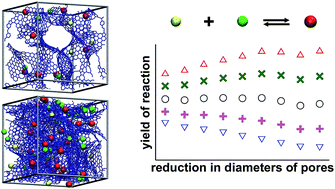
This work has presented the results of a rather extensive Monte Carlo study concerning the effects of confinement on the reactions taking place in the pores of activated carbons. We have considered here three simple model reactions: isomerisation, dimerisation and synthesis, and investigated how the changes in the carbon porosity, the values of the equilibrium constant, and the energetic parameters of the reacting molecules influence the chemical equilibria. The obtained results show that the main factors affecting the reaction equilibria in pores are the latest ones. When the adsorption energy of the product molecules is higher than that of the reactants, the confinement causes a rise in the reaction yield. In the opposite situation (preferential adsorption of the reactants), the product mole fraction inside the pores is lower than in the bulk phase. It has been shown that the porous structure of activated carbons plays a very important role. The reduction of pore diameters may either increase or decrease the reaction yield, depending on the relative adsorption energy of the reactants and the products. If the product molecules are bigger than the reactant molecules, the presence of pores accessible for the reactant molecules, but inaccessible for the product, causes additional reduction of the reaction yield regardless of the magnitudes of the energetic parameters of the reacting species.


 Please wait while we load your content...
Please wait while we load your content...