Copper powder-catalyzed chelation-assisted cascade reaction of o-chloroarylacetic acids with amines under solvent- and ligand-free conditions: synthesis of oxindoles†
Abstract
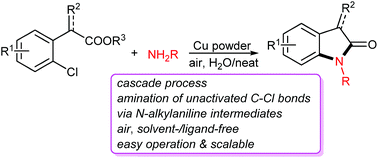
An efficient method to construct oxindole scaffolds from o-chloroarylacetic acids/esters with amines has been explored. This cascade protocol involves the in situ generation of o-aminoarylacetic acid derivatives by the copper powder catalyzed and weak O-chelation assisted Ullmann amination of unactivated C–Cl bonds under air, and solvent-/ligand-free conditions followed by annulative N-acylation.


 Please wait while we load your content...
Please wait while we load your content...