P450 monooxygenase ComJ catalyses side chain phenolic cross-coupling during complestatin biosynthesis†
Abstract
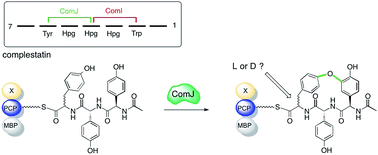
Complestatin is a non-ribosomal heptapeptide belonging to the glycopeptide antibiotic family that displays anti-complement, anti-HIV integrase, neuroprotective, anti-apoptotic, and antibacterial activities. Development of complestatin as a pharmaceutical agent and biological probe has been stymied by the difficulty in isolating the molecule from its natural source, Streptomyces lavendulae, as well as by the uneconomical and environmentally unfriendly nature of current total synthetic routes. Of particular challenge to the organic chemist is the installation of the biaryl and aryl–ether–aryl linkages that provide complestatin with the structural rigidity responsible for its potent pharmacological properties. Herein, we demonstrate that ComJ, a P450 monooxygenase from the complestatin biosynthetic gene cluster, can catalyse phenolic cross-linking of amino acid side chains in vitro. ComJ acts with high efficiency and low substrate stereoselectivity, a finding which paves the way towards the use of ComJ as a biocatalyst for the chemo-enzymatic synthesis of complestatin and other related molecules. The ability of ComJ to accept peptides of alternative stereochemistries raises intriguing questions about the evolutionary origins of glycopeptide antibiotic biosynthesis and the capacity of S. lavendulae to produce different conformations of complestatin.


 Please wait while we load your content...
Please wait while we load your content...