Mono- and double carbonylation of aryl iodides with amine nucleophiles in the presence of recyclable palladium catalysts immobilised on a supported dicationic ionic liquid phase†
Abstract
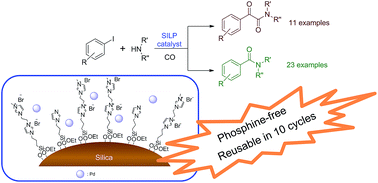
Silica modified with organic dicationic moieties proved to be an excellent support for palladium catalysts used in the aminocarbonylation of aryl iodides. By an appropriate choice of the reaction conditions, the same catalyst could be used for selective mono- or double carbonylations leading to amide and α-ketoamide products, respectively. The best catalyst could be recycled for at least 10 consecutive runs with a loss of palladium below the detection limit. By the application of the new support, efficient catalyst recycling could be achieved under mild reaction conditions (under low pressure and in a short reaction time). Palladium-leaching data support a mechanism with dissolution—re-precipitation of the active palladium species.


 Please wait while we load your content...
Please wait while we load your content...