Benzene-glycol nucleic acid (BGNA)–DNA chimeras: synthesis, binding properties, and ability to elicit human RNase H activity†
Abstract
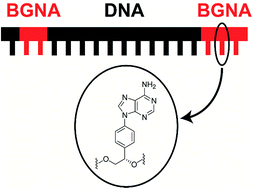
This paper describes the synthesis and properties of benzene-glycol nucleic acid (BGNA)–DNA chimeras containing four nucleoside analogs – thymidine, cytidine, adenosine, and guanosine – with a base-benzene-glycol structure. We found that the BGNA–DNA chimeras are able to form thermally and thermodynamically stable duplexes with complementary RNAs, and have base-discriminating abilities. The BGNA–DNA chimeras were 20-fold more stable in a buffer containing 30% bovine serum than unmodified DNA. Furthermore, BGNA–DNA chimera/RNA duplexes were found to be good substrates for human RNase H. Thus, BGNA–DNA chimeras are good candidates for the development of therapeutic antisense molecules.

 Please wait while we load your content...
Please wait while we load your content...