Fatty acid eutectic mixtures and derivatives from non-edible animal fat as phase change materials†
Abstract
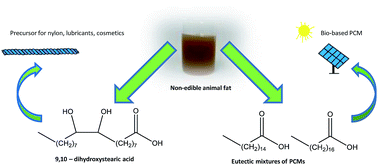
A set of compounds from non-edible fat waste was prepared and their thermal behavior was studied. The fat was hydrolyzed and crystallized in a simple and robust process to yield palmitic acid–stearic acid (PA–SA) mixtures. The PA–SA mass ratios determined by GC-FID (gas chromatography-flame ionization detection) were similar to those reported for eutectic mixtures of PCMs (phase change materials). DSC (differential scanning calorimetry) results indicated that the melting and solidification temperatures were around 55 °C and 52 °C and the latent heat of the crystallized fractions measured was around 180 kJ kg−1. The thermal cycling reliability of the eutectic mixtures was also tested during 1000 melting/freezing cycles. The loss in melting and solidification enthalpies was below 14% in all mixtures showing a promising behavior for PCM applications. Additionally, the unsaturated fatty acids were recovered and transformed to threo-9,10-dihydroxystearic acid (DHSA) and some of their inorganic salts, which were analyzed by FT-IR (Fourier transform-infrared spectroscopy) and tested for the first time using the DSC technique.


 Please wait while we load your content...
Please wait while we load your content...