Synthesis of a cyclopentadienyl(imino)stannylene and its direct conversion into halo(imino)stannylenes†
Abstract
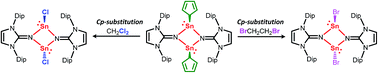
The reaction of stannocene Cp2Sn with iminolithium LiNIPr (NIPr = bis(2,6-diisopropylphenyl)imidazolin-2-iminato) afforded the dimeric cyclopentadienyl(imino)stannylene [(η1-Cp)SnNIPr]2 (1). Compound 1 exhibits unexpected reactivity towards haloalkanes. The high-yield conversion of 1 into chlorostannylene [ClSnNIPr]2 (2) and bromostannylene [BrSnNIPr]2 (3) were accomplished by treatment with dichloromethane or 1,2-dibromoethane, respectively, through a Cp-substitution reaction.

 Please wait while we load your content...
Please wait while we load your content...