Direct arylation of benzoazoles with aldehydes utilizing metal–organic framework Fe3O(BDC)3 as a recyclable heterogeneous catalyst†
Abstract
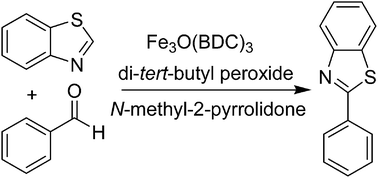
A metal–organic framework Fe3O(BDC)3 was synthesized and used as a recyclable heterogeneous catalyst for the direct arylation of azoles with benzaldehydes. Following this protocol, inexpensive and commercially available benzaldehydes could act as an aryl source for the transformation, replacing the aryl halides normally employed in the conventional cross-coupling reactions. The catalyst disclosed higher catalytic productivity for the formation of aryl-substituted azoles than various MOFs and diverse homogeneous iron catalysts. The conversion was considerably regulated by the solvent and the oxidant, and the combination of N-methyl-2-pyrrolidone with di-tert-butyl peroxide offered the best yield. Heterogeneous catalysis was verified for the reaction, and the production of aryl-substituted azoles was only feasible in the presence of the iron framework. It was possible to reuse the catalyst for the direct arylation of azoles with aldehydes while its catalytic activity was preserved for multiple cycles. To our best judgment, this direct arylation of azoles with aldehydes utilizing solid catalysts has not been previously reported.

 Please wait while we load your content...
Please wait while we load your content...