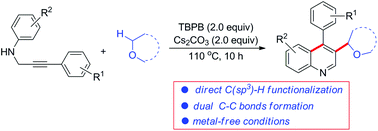
tert-Butyl peroxybenzoate mediated formation of 3-alkylated quinolines from N-propargylamines via a cascade radical addition/cyclization reaction†
Abstract
A tert-butyl peroxybenzoate (TBPB) mediated direct functionalization of N-propargyl aromatic amine derivatives with ethers has been developed. This procedure provides a new strategy toward the synthesis of 3-alkylated quinolines in one step via a domino radical addition/cyclization reaction under metal-free conditions.


 Please wait while we load your content...
Please wait while we load your content...