Mechanistic insight into the C7-selective C–H functionalization of N-acyl indole catalyzed by a rhodium complex: a theoretical study†
Abstract
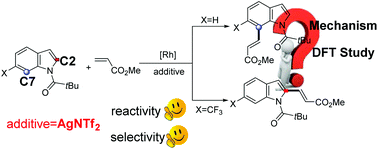
Herein, the reaction mechanisms for the rhodium-catalyzed regioselective C7-functionalization of N-acyl indole are theoretically investigated by employing density functional theory (DFT) calculations. The role of the additive AgNTf2 in enhancing the reactivity and selectivity for the C7–H functionalization of N-acyl indole is clarified by calculations. The origins of the effect of the substituent group at the 6-position of N-acyl indole on the regioselectivity are explored by analyzing the electron and steric effects. The 7-olefination product is preferred for the H-substituted substrate (reaction A), which is attributed to the greater nucleophilicity of the C7 atom in comparison to that of the C2 atom. This facilitates the subsequent electrophilic attack of the Rh center. In reaction B with the CF3-substituted substrate, the 2-olefination product is preferred over the 7-olefination product mainly because of the stronger steric effects in TS2(C7)F compared with that in TS2(C2)F.


 Please wait while we load your content...
Please wait while we load your content...