Practical synthesis of enantiopure benzylamines by catalytic hydrogenation or transfer hydrogenation reactions in isopropanol using a Ru-pybox catalyst†
Abstract
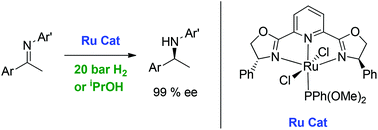
The screening of a family of complexes of the formula [RuCl2(R-pybox)(L)] (R-pybox = Ph-, iPr- or indane-pybox; L = monodentate P, N or C ligand) in the enantioselective hydrogenation of N-aryl imines indicates a strong influence of the R-pybox substituents and the L ligand in the process. A comparison indicates that the best results are obtained with the complex [RuCl2(Ph-pybox)(PPh(OMe)2)] which provided values of 99% ee for the reduction of several imines derived from aryl alkyl ketones. It is worth noting that this complex is capable of reducing the mentioned imines under transfer hydrogenation conditions using isopropanol as a hydrogen donor with equally high enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...