Ni(i)–Ni(iii) vs. Ni(ii)–Ni(iv): mechanistic study of Ni-catalyzed alkylation of benzamides with alkyl halides†
Abstract
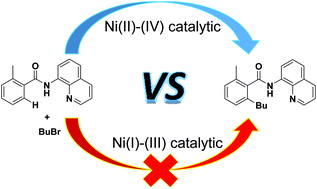
Nickel-catalyzed C–H bond activation has attracted significant attention for the construction of C–C bond frameworks. We report density functional theory investigations into the mechanism of nickel-catalyzed alkylation of benzamides with alkyl halides. Both the Ni(I)–Ni(III) and Ni(II)–Ni(IV) catalytic cycles were considered. The theoretical study indicated that the most feasible mechanism involved a Ni(II)–Ni(IV) catalytic cycle with four main steps: (i) N–H bond activation and (ii) C–H bond activation through the concerted metalation–deprotonation pathway, (iii) oxidative addition of BuBr to give a high-valent Ni(IV) complex, and (iv) C–C reductive elimination to generate the product and the active catalyst. The rate-determining step of the favored pathway is the oxidative addition, leading to the generation of a Ni(IV) intermediate. In addition, the present study casts light on the role of PPh3, which accelerates the cleavage of N–H bond. Frontier molecular orbital theory and natural population analysis were employed to explain the effect of the phosphine ligand on the structure of the Ni complex.


 Please wait while we load your content...
Please wait while we load your content...