Synthesis of sulfonated naphthols via C–H bond functionalization with the insertion of sulfur dioxide†
Abstract
A three-component reaction of naphthols, sulfur dioxide, and aryldiazonium tetrafluoroborates catalyzed by iron(III) chloride through a radical process via direct C–H functionalization is developed, leading to diverse sulfonated naphthols in good yields. Arylsulfonyl radicals act as the key intermediates during the reaction process, and the presence of iron(III) chloride facilitates the formation of a naphthol radical in the reaction through a single electron transfer. Several sensitive functional groups including nitro, halo, cyano, hydroxy, and ester are all compatible under the conditions.
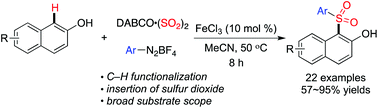


 Please wait while we load your content...
Please wait while we load your content...