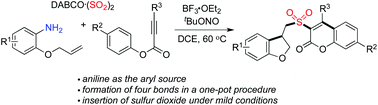
Synthesis of 3-(((2,3-dihydrobenzofuran-3-yl)methyl)sulfonyl) coumarins through the reaction of 2-(allyloxy)anilines, sulfur dioxide, and aryl propiolates†
Abstract
A facile route to 3-((2,3-dihydrobenzofuran-3-yl)methyl)sulfonyl coumarins through the reaction of 2-(allyloxy)anilines, DABCO-bis(sulfur dioxide), and aryl propiolates is achieved. During the reaction process, a 2-(allyloxy)aryl radical would be generated in situ, which would undergo the intramolecular addition of a double bond to afford an alkyl radical intermediate. Further insertion of sulfur dioxide would produce an alkylsulfonyl radical. The subsequent combination with aryl propiolates would provide sulfonyl-bridged dihydrobenzofuran and coumarin derivatives through radical cyclization and rearrangement.


 Please wait while we load your content...
Please wait while we load your content...