Metal-free annulation of β-acylamino ketones: facile access to spirooxazolines and oxazolines via oxidative C–O bond formation†
Abstract
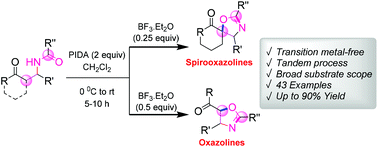
A metal-free annulation reaction of β-acylamino ketone derivatives has been reported for the synthesis of a group of functionalized spirooxazolines and oxazolines in good to excellent yields. The reaction proceeds via phenyliodine(III) diacetate (PIDA)-mediated oxidative C–O bond formation in the presence of BF3–OEt2. The mild reaction conditions, broad substrate scope, simple execution and synthetic potential of the products make this novel protocol very attractive.


 Please wait while we load your content...
Please wait while we load your content...