The regioselective synthesis of 2-phosphinoylindoles via Rh(iii)-catalyzed C–H activation†
Abstract
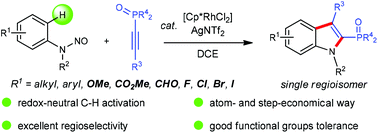
The redox-neutral annulation of N-nitrosoanilines with 1-alkynylphosphine oxides under rhodium catalysis was achieved. This step-economical protocol affords 2-phosphinoylindoles in excellent regioselectivity with good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...