Stereospecific C–H activation as a key step for the asymmetric synthesis of various biologically active cyclopropanes†
Abstract
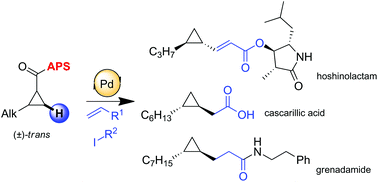
A rapid and efficient synthesis of hoshinolactam, an enantiopure cyclopropane containing natural product, is described. This strategy is based on stereospecific C(sp3)–H activation via unprecedented olefination on the cyclopropane core. The use of a stereogenic and easily recyclable directing group (S)-2-(p-tolylsulfinyl)aniline (APS) which was originally developed by our group allows obtention of diastereomerically pure products in high yields. Optically pure cyclopropanes are subsequently converted into enantiomerically pure targeted natural products. Furthermore a closely related synthetic scenario is employed to build up precursors of grenadamide and cascarillic acid.
- This article is part of the themed collection: Synthetic Approaches to Natural Products via Catalytic Processes


 Please wait while we load your content...
Please wait while we load your content...