A thiol-free synthesis of alkynyl chalcogenides by the copper-catalyzed C–X (X = S, Se) cross-coupling of alkynyl carboxylic acids with Bunte salts†
Abstract
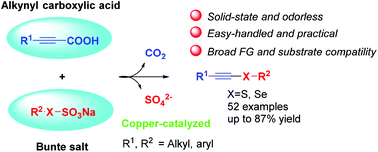
An efficient copper-catalyzed C–X (X = S, Se) decarboxylative coupling of alkynyl carboxylic acids with Bunte salts has been developed. The process is amenable to various alkynyl carboxylic acids and Bunte salts and is compatible with a wide range of functional groups. In addition, this catalytic reaction could also be performed with seleno Bunte salts and alkynyl carboxylic acids under the same conditions, giving the alkynyl selenosulfides in good yields. Importantly, this route avoids the use of odorous thiol or selenol starting materials.


 Please wait while we load your content...
Please wait while we load your content...