Highly mono-selective ortho-methylthiolation of benzamides via cobalt-catalyzed sp2 C–H activation†
Abstract
A highly mono-selective ortho-methylthiolation of benzamides was achieved via Co-catalyzed coupling of benzamides with DMSO. The reaction employs an 8-aminoquinoline group as the bidentate directing group and DMSO as the methylthiolation source. Instead of a mixture of mono- and di-alkylthio-substituted products obtained by previously reported procedures, our reaction gave the mono-methylthioethers only. Another notable feature of this newly developed protocol is that acrylamides can also be efficiently mono-methylthiolated.
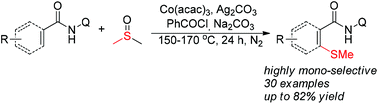


 Please wait while we load your content...
Please wait while we load your content...