Photoredox-catalyzed cascade addition/cyclization of N-propargyl aromatic amines: access to 3-difluoroacetylated or 3-fluoroacetylated quinolines†
Abstract
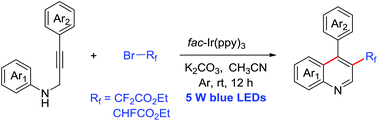
3-Difluoroacetylated quinolines and 3-fluoroacetylated quinolines were synthesized via a visible-light-induced cascade addition/cyclization of N-propargyl aromatic amines with ethyl bromodifluoroacetate or ethyl bromofluoroacetate catalyzed by fac-Ir(ppy)3 under mild conditions. For the substrates bearing various substituents on the aniline ring and benzene ring, the reaction proceeded smoothly to give the corresponding difluoroacetylated or fluoroacetylated quinolines in moderate to high yields.


 Please wait while we load your content...
Please wait while we load your content...