A copper(i)-catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines: enantioselective synthesis of 3-substituted 3-aminooxindoles†
Abstract
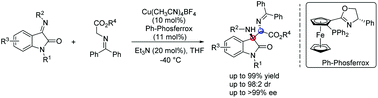
A Cu(I)/Ph-Phosferrox complex catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines has been described. This strategy can provide direct access to chiral 3-substituted 3-aminooxindoles bearing vicinal quaternary–tertiary carbon stereocenters in high yields (up to 99%), excellent enantioselectivities (up to >99% ee) and moderate to high diastereoselectivities (up to 98 : 2 dr).
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...