Chemoselective C(α)–C(β) bond cleavage of saturated aryl ketones with amines leading to α-ketoamides: a copper-catalyzed aerobic oxidation process with air†
Abstract
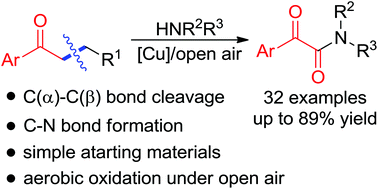
An unprecedented selective C(α)–C(β) bond cleavage of simple saturated aryl ketones was developed. The corresponding α-ketoamides, ubiquitous structural units in a variety of natural products, were constructed from copper-catalyzed aerobic oxidative C–C bond cleavage and amidation of ketones with amines under air. And this reaction featured simple starting materials and a broad substrate scope. Moreover, no obvious limitation was observed in terms of a gram-scale application. In addition, a plausible reaction pathway was proposed based on mechanism studies.


 Please wait while we load your content...
Please wait while we load your content...