Rearranged limonoids with unique 6/5/6/5 tetracarbocyclic skeletons from Toona ciliata and biomimetic structure divergence†
Abstract
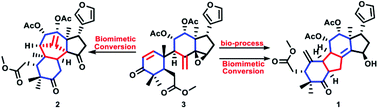
Tooniliatone A (1), a novel limonoid with an unprecedented 6/5/6/5 tetracarbocyclic skeleton was isolated from Toona ciliata Roem. var. yunnanensis. Its rearranged ring-seco backbone was proposed to biosynthetically originate from co-isolated known toonacilin (3) via a rare electron-deficient alkene electrophilic cyclization and 1,2-alkyl shift sequence. The acid-catalyzed biomimetic conversion of 3 not only successfully afforded 1 but also another novel 6/5/6/5 tetracarbocyclic skeleton, tooniliatone B (2). The priority of 2 in this biomimetic conversion, the existence of 1 and the absence of 2 in the crude extract suggested the biosynthetic selectivity of 1 from 3. The divergent biomimetic sequence, based on a carbocation from an epoxide moiety, provided an inspiration for constructing complex polycyclic carbon skeletons.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...