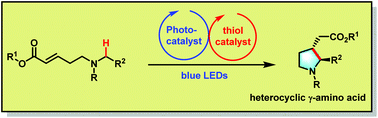
Photoredox organocatalytic α-amino C(sp3)–H functionalization for the synthesis of 5-membered heterocyclic γ-amino acid derivatives†
Abstract
A 5-membered heterocyclic γ-amino acid scaffold possesses a wide range of bioactivities. Herein, we report a photoredox organocatalytic, highly selective α-amino C(sp3)–H bond functionalization to generate a reactive α-amino radical, which undergoes conjugate addition to Michael acceptors, providing an elegant intramolecular access to cyclic γ-amino acid analogues in satisfactory yields.


 Please wait while we load your content...
Please wait while we load your content...