Visible light photoredox catalyzed semisynthesis of the analogues of maclekarpine E: a series of 6-vinyl substituted dihydrobenzophenanthridine alkaloids†
Abstract
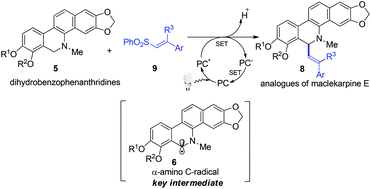
A visible light promoted vinylation of N-methyl 5,6-dihydrobenzophenanthridine was developed to synthesize the analogues of maclekarpine E. In this photoredox neutral radical coupling reaction, an α-amino C-radical generated at the C-6 of N-methyl 5,6-dihydrobenzophenanthridine was the key intermediate. The subsequent radical addition of vinyl sulfones with the α-amino C-radical followed by elimination of a sulfinyl radical gave the target compounds in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...