B2(OH)4-mediated one-pot synthesis of tetrahydroquinoxalines from 2-amino(nitro)anilines and 1,2-dicarbonyl compounds in water†
Abstract
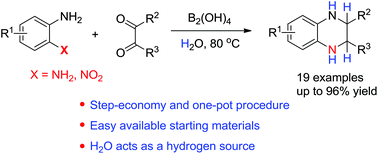
A practical one-pot synthesis of tetrahydroquinoxalines from readily available 2-amino(nitro)anilines and 1,2-dicarbonyl compounds mediated by diboronic acid with water as both a solvent and a hydrogen donor under metal-free conditions has been discovered. The deuterium labeling experiments with D2O were also performed to demonstrate that water acts as the hydrogen donor.


 Please wait while we load your content...
Please wait while we load your content...