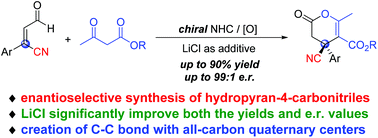
NHC-catalyzed enantioselective synthesis of dihydropyran-4-carbonitriles bearing all-carbon quaternary centers†
Abstract
A carbene-catalyzed oxidative reaction for the synthesis of dihydropyran-4-carbonitriles bearing all-carbon quaternary centers was developed. The use of a LiCl additive significantly improved both the reaction efficiency and stereo-selectivities, and the carbonitrile products were obtained in good yields and excellent er values.


 Please wait while we load your content...
Please wait while we load your content...