Disilanes as oxygen scavengers and surrogates of hydrosilanes suitable for selective reduction of nitroarenes, phosphine oxides and other valuable substrates†
Abstract
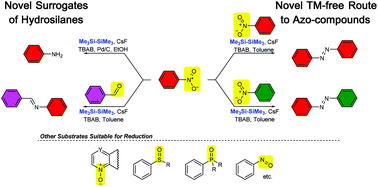
In this report, we demonstrate that the reaction of nitroarenes with hexamethyldisilane under various conditions affords a different range of compounds with excellent selectivity. In particular, the reaction of nitroarenes with hexamethyldisilane using a CsF/TBAB/toluene system provides suitable azo compounds, while in the presence of a CsF/TBAB/[Pd]/EtOH system hexamethyldisilane acts as a novel surrogate of gaseous trimethylsilane, thus, reducing nitroarenes to corresponding anilines. The synthetic value of the developed methodology was further extended by the reduction of a wide range of substrates including N-oxides, sulfoxides, phosphine oxides etc.


 Please wait while we load your content...
Please wait while we load your content...