Highly enantioselective Rh/chiral sulfur-olefin-catalyzed arylation of alkyl-substituted non-benzofused cyclic N-sulfonyl ketimines†
Abstract
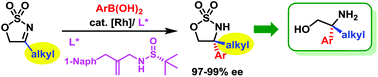
A highly enantioselective Rh-catalyzed addition of arylboronic acids to non-benzofused five-membered cyclic N-sulfonyl alkyl-substituted ketimines has been developed. With simple chiral sulfur-olefin as the ligand, the reaction allows convenient access to a variety of sulfamidates with uniformly excellent enantioselectivities (97–99% ee). The synthetic utility of this protocol was exemplified by rapid asymmetric construction of tetrasubstituted chiral β-amino alcohols and their derivatives.


 Please wait while we load your content...
Please wait while we load your content...