Rapid construction of the 6/6/5 tricyclic framework via a tandem radical cyclization reaction and its application to the synthesis of 5-epi-7-deoxy-isoabietenin A†
Abstract
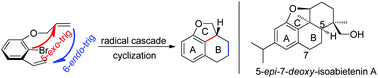
A tandem radical cyclization reaction towards the 6/6/5 fused tricyclic skeleton, which exists in numerous natural products, was developed in modest to good yields. In this transformation, two C–C bonds and two rings were formed successively via a tandem 5-exo-trig/6-endo-trig cyclization reaction. 5-epi-7-deoxy-Isoabietenin A was also synthesized efficiently via this strategy.
- This article is part of the themed collection: Synthetic Approaches to Natural Products via Catalytic Processes


 Please wait while we load your content...
Please wait while we load your content...