Ir(iii)-Catalyzed site-selective amidation of azoxybenzenes and late-stage transformation
Abstract
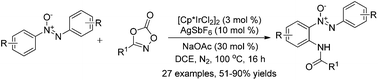
An efficient ortho C–H amidation of azoxybenzenes with 1,4,2-dioxazol-5-ones under Ir(III)-catalysis has been developed. This simple amidation reaction provides a new protocol for the diversification of azoxybenzenes, and affords the aminated products in moderate to good yields. Meanwhile, an investigation of the late-stage transformation of products is also presented in this text.


 Please wait while we load your content...
Please wait while we load your content...