Facile synthesis of 1-aminoindoles via Rh(iii)-catalysed intramolecular three-component annulation†
Abstract
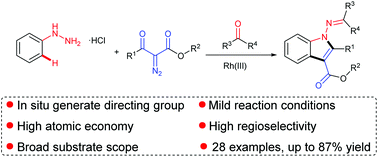
The facile synthesis of various 1-aminoindoles from readily available aryl hydrazines, diazo compounds and ketones via rhodium catalysed C–H activation has been developed. The reaction was carried out under mild reaction conditions using hydrazone as a directing group, which was formed by the in situ condensation of hydrazines and ketones. This protocol exhibits a broad substrate scope and step economy, releasing N2 and H2O as the byproducts, obviating the need for external oxidants and pre-functionalized substrates.


 Please wait while we load your content...
Please wait while we load your content...