An enantioselective conjugate addition reaction of 3-substituted benzothiophen-2-ones and 2-phthalimidoacrylates†
Abstract
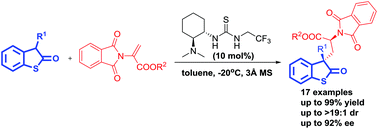
A highly enantioselective conjugate addition reaction of 3-substituted benzothiophen-2-ones to 2-phthalimidoacrylates has been developed using a bifunctional tertiary-amine thiourea catalyst. A number of chiral 3-substituted benzothiophen-2-one compounds were obtained with excellent yields (up to 99%) and very good stereoselectivities (up to >19 : 1 dr and up to 92% ee). The reaction was proved to be an efficient strategy for the synthesis of enantioenriched α-amino acid derivatives with 1,3-nonadjacent stereogenic centers.


 Please wait while we load your content...
Please wait while we load your content...