Diastereoselective building up polycyclic tetrahydrofurans via tandem annulation of 1,n-enynes with aliphatic acids†
Abstract
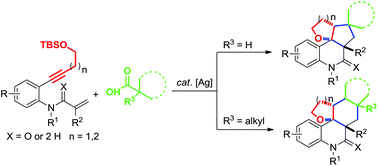
A silver-catalyzed tandem annulation of aniline-linked 1,7-enynes with aliphatic acids has been reported. This synergistic tandem annulations allow the straightforward and efficient building up various highly diastereoselective polycyclic tetrahydrofurans. Primary and secondary acids undergo decarboxylation/cascade radical addition/α-C(sp3)–H cleavage/cycloalkylation/O-trapping to generate oxygenous [6.6.6.5] fused products. While tertiary acids undergo decarboxylation/cascade radcial addition/β-C(sp3)–H cleavage/O-trapping to give oxygenous [6.6.6.6] fused products.


 Please wait while we load your content...
Please wait while we load your content...