Rhodium(iii)-catalyzed synthesis of indanones via C–H activation of phenacyl phosphoniums and coupling with olefins†
Abstract
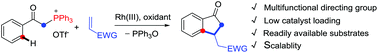
Phosphonium ylide acts as an efficient bifunctional directing group in Rh(III)-catalyzed C–H activation of arenes and oxidative coupling with activated olefins, leading to facile construction of indanones via a sequence of oxidative olefination and carboannulation. The phosphonium moiety functions as an oxophilic group, and dephosphination triggers a nucleophilic cyclization.


 Please wait while we load your content...
Please wait while we load your content...